Neuronox®
Global Botulinum toxin type A, Neuronox®
Neuronox® is Korea’s first and the fourth botulinum toxin type A product in the world, launched in 2006.
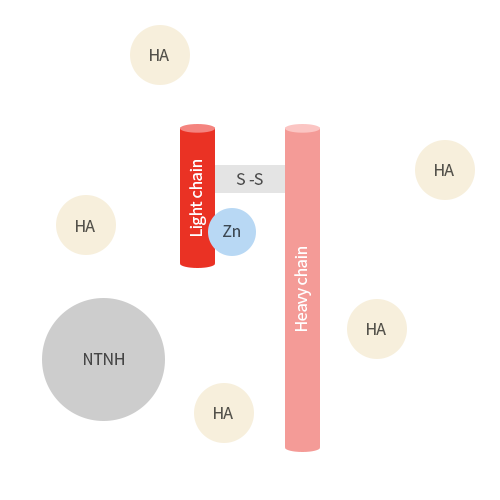
Botulinum toxin is a neurotoxin, released from Clostridium botulinum, which offers improvement of facial wrinkles by
paralyzing muscles.

Neuronox® is sold worldwide under different brand names including

Botulinum toxin is consist of botulinum neurotoxin and non-toxic proteins.
Cause of wrinkles ¹
1. Repeated facial expressions
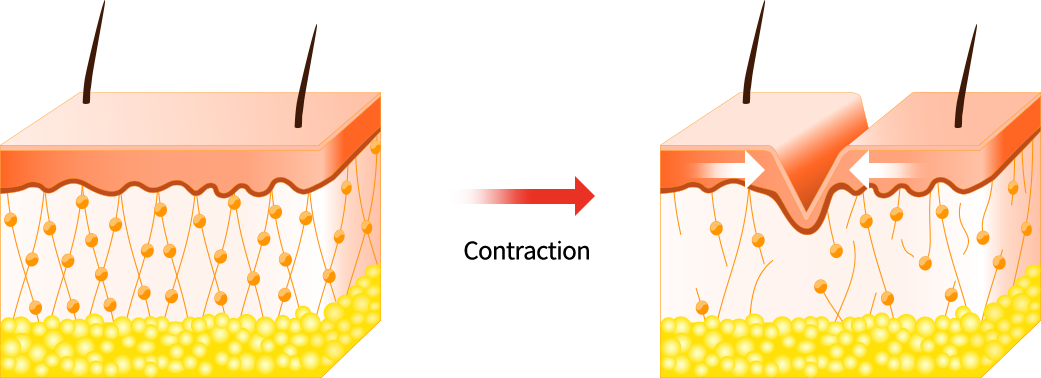
Facial movements and expressions, such as squinting or smiling, lead to fine lines and wrinkles.
Each time you use a facial muscle, a groove forms beneath the surface of the skin.
And as skin ages, it loses its flexibility and is no longer able to spring back in place.
These grooves then become permanent features on your face.
2. Age
As you get older, your skin naturally becomes less elastic and more fragile.
Decreased production of natural oils dries your skin and makes it appear more wrinkled.
Fat in the deeper layers of your skin diminishes. This causes loose, saggy skin and more-pronounced lines and crevices.

Why Neuronox®?
1. C. botulinum (clostridium botulinum) Hall A hyper, the strain of Neuronox®, is a suitable for the therapeutic agent.²
1) C. botulinum Hall A hyper is a hypertoxin producer that can produce toxins in a simple growth medium
and is thus a strain suitable for treatment.²
2) C. botulinum Hall A hyper of Medytox (DQ409059) has the same origins of strain as Allergan (AF488749 etc.).²
Fast production  |
Produces toxins rapidly over the same time period. |
|---|---|
Increased production  |
Produces the most toxins over the same time period. |
Hypertoxigenic strain  |
Produces toxins with the highest toxicity when cultivated in the same growth medium (TPM). |
 : The kinetics of botulinum toxin gene expression have been investigated in C. botulinum type A strains 62A, Hall A-hyper, and type A(B) strain NCTC 2916 during the growth cycle.
The analyses were performed in TPGY(tryptcase peptone glycose yeast extract) and type A TPM(toxin production media).
: The kinetics of botulinum toxin gene expression have been investigated in C. botulinum type A strains 62A, Hall A-hyper, and type A(B) strain NCTC 2916 during the growth cycle.
The analyses were performed in TPGY(tryptcase peptone glycose yeast extract) and type A TPM(toxin production media).
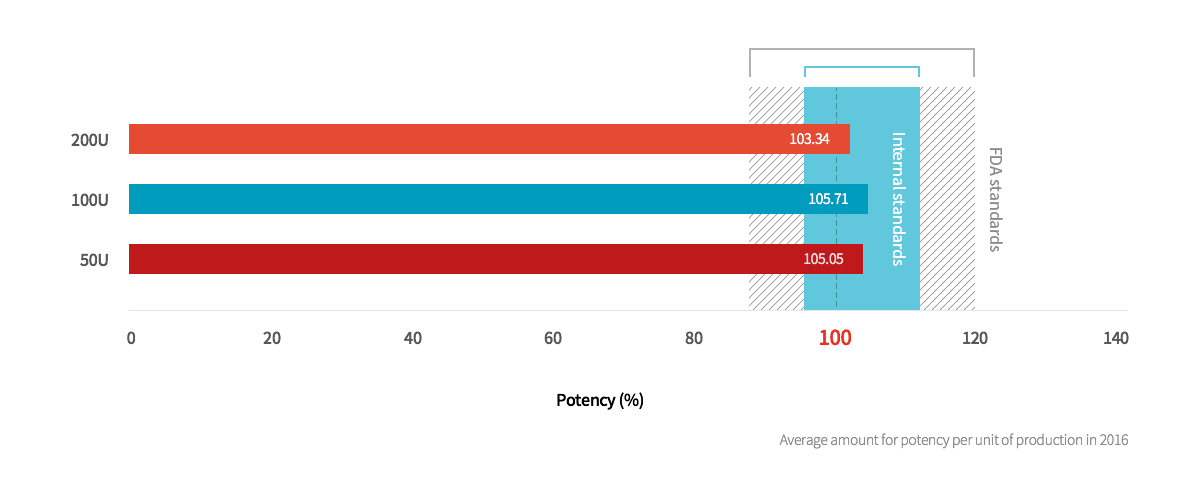
2. Neuronox® is produced under strict administrative standards with stable potency levels
In general, the global biological product testing standard for potency is 85% ~ 120% for standardized goods,
but Medytox produces Neuronox® under stricter standards.
 Potency of Neuronox® based on units
Potency of Neuronox® based on units 
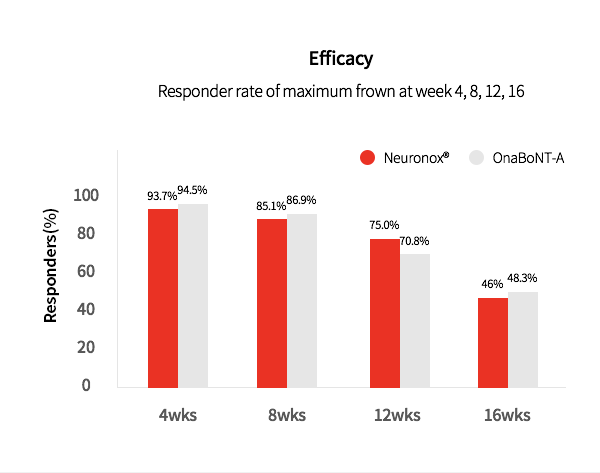
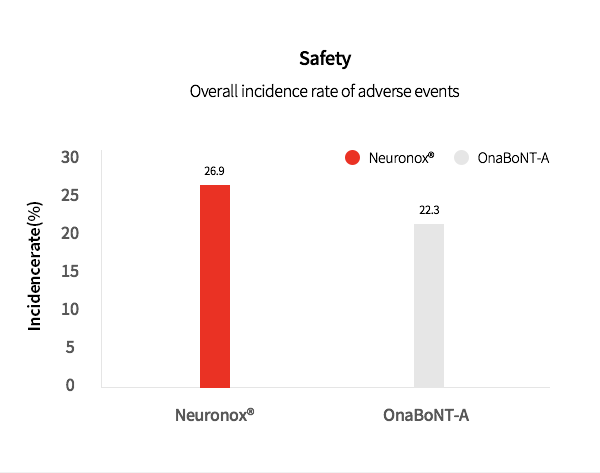
3. Neuronox® was effective and safe for treatment of moderate to severe glabellar lines
Neuronox® is as effective as OnaBoNT-A in reducing moderate to severe glabellar lines, while also being safe to use.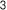
Clinical studies
-
1. Moderate to severe glabellar lines
Chong Hyun Won et al. Efficacy and safety of a novel botulinum toxin type A product for the treatment of moderate to severe glabellar lines: a randomized, double-blind, active-controlled multicenter study, Dermatol Surg. 2013 Jan;39(1 Pt 2):171-8.
-
2. Blepharospasm
Jin Sook Yoon et al. Double-Blind, Randomized, Comparative Study of Meditoxin® Versus Botox® in the Treatment of Essential Blepharospasm, Korean J Ophthalmol. 2009 Sep; 23(3): 137–141.
-
3. Pediatric cerebral palsy
Keewon Kim et al. Neuronox versus BOTOX for spastic equinus gait in children with cerebral palsy: a randomized, double-blinded, controlled multicentre clinical trial, Dev Med Child Neurol. 2011 Mar;53(3):239-44.
-
4. Muscle spasticity
Han Gil Seo et al. Neuronox versus BOTOX in the Treatment of Post-Stroke Upper Limb Spasticity: A Multicenter Randomized Controlled Trial, PLoS One. 2015 Jun 1;10(6):e0128633. doi: 10.1371/journal.pone.0128633. eCollection 2015.
Neuronox® information
- Mayo Clinic. Wrinkles - Symtoms & causes. available at: https://www.mayoclinic.org/diseases-conditions/wrinkles/symptoms-causes/syc-20354927 accessed Feb, 2023.
- Dermatol Surg 2013; 39: 165-170.
- Dermatol Surg 2013; 39(1 Pt 2): 171-8.
- Anaerobe 2004; 10: 321 -333.
- Notification of MFDS 2016-111.
- Data on file